Written by:
Allan Huysamen Technical Manager Adjuvants
A comprehensive understanding of the quality of a specific water source destined for tank-mix applications can be the difference between the efficient and inefficient use of agrochemicals.
Factors affecting agrochemical efficiency
The quality of a specific body of water is defined by its chemical properties, which are directly related to the substances which are dissolved and suspended in it. Although multiple sources of water are used for crop irrigation, including rainfall, the overwhelming majority of water used for tank-mix purposes is extracted from groundwater and surface water, such as aquifers and dams or rivers.
It is important to keep in mind that each water source has unique properties and chemistry, due to its interactions with the atmosphere, soils, mineral rocks and organisms.
The requirements for water to be used for irrigation purposes are different to those for tank-mix applications, as the latter serves mainly as a carrier solution for agrochemicals to be applied to the leaf surfaces of crops. It is thus important to ensure that the water quality is as high as possible or that appropriate corrections are made, in order to maximise the efficacy of the active ingredients in solution.
Organic contaminants, such as soil particles and decomposed plant matter, can decrease the efficacy of products being administered, especially those which possess a high soil organic carbon sorption coefficient (Koc).
Agrochemicals which exhibit this property include glyphosate, diquat and paraquat, and the presence of organic carbon in the water source can irreversibly bind these molecules, resulting in them being unavailable for foliar uptake. Inorganic contaminants are more often than not present in relatively high concentrations within surface and groundwater, as a result of dissolved mineral salts from the interaction of water with mineral rocks. Acidification of water, due to the absorption of carbon dioxide from the atmosphere, allows for the acceleration of this process and thereby increases the concentration of salt deposits. The hardness and alkalinity of a particular source are determined by these concentrations and have major implications for certain groups of agrochemical products. For example, cations such as Ca2+ and Mg2+ bind and antagonise negatively charged herbicides such as glyphosate by stunting their uptake and preventing binding to their systemic targets.
Analysis as a solution
Valuable information can be gained by analysing water to be used for spray solutions, allowing for informed decisions to be made to maximise product efficiency.
Constituting part of the Agri Technovation suite of services is the ITEST™️ WATER – Spray Solution Properties service, which aims to provide valuable insight into the water quality of a particular source. With the submission of each sample, the water is analysed in our laboratory with respect to major quality parameters and experimentally determined suggestions for volumes of water quality correction adjuvants are provided.
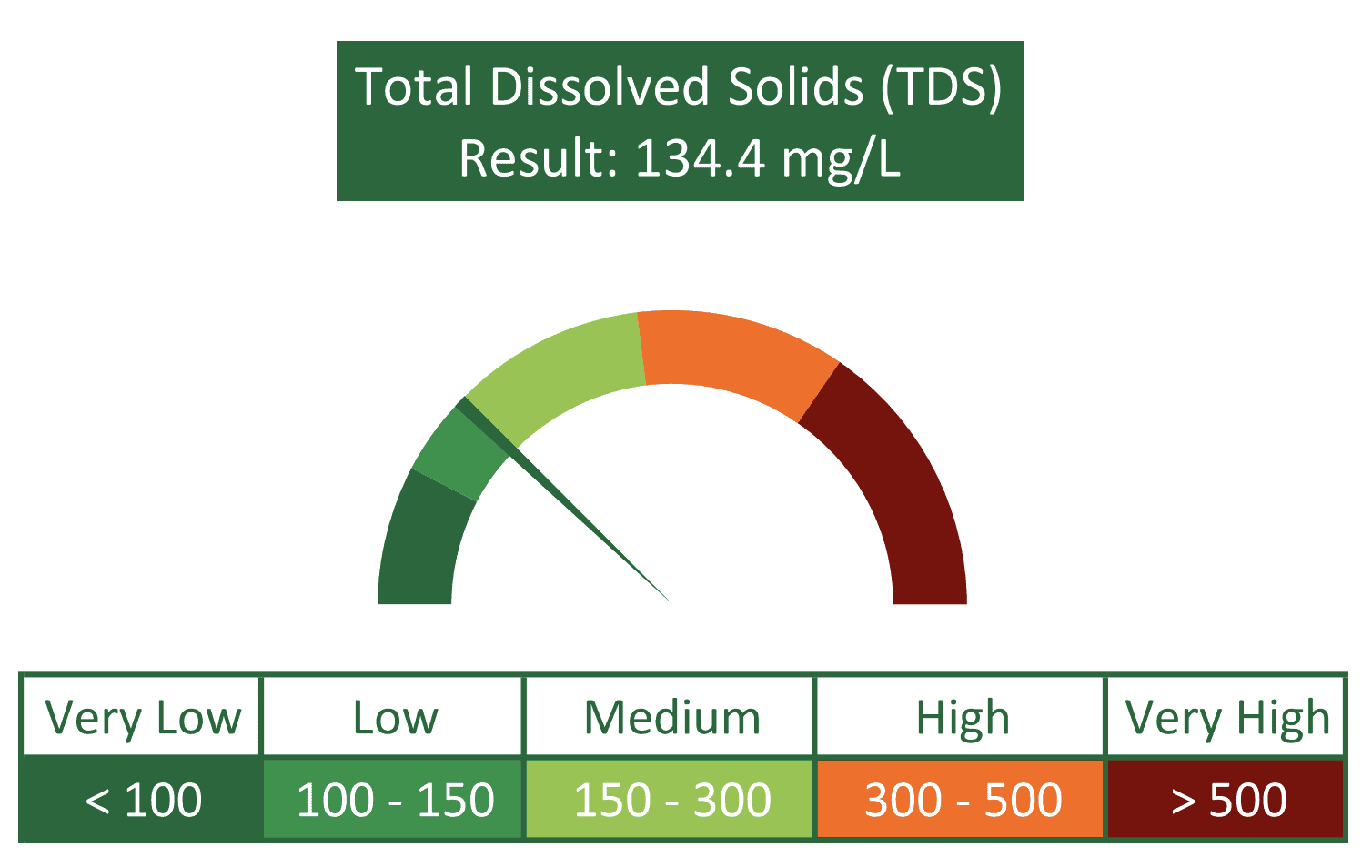
Each water sample is assessed for turbidity and total suspended solids, which acts as an indication for potential levels of organic carbon in the water and provides caution when crop protection products with a high Koc are considered. Other major water quality parameters which are important to take into consideration for spray solutions include alkalinity, pH, electrical conductivity (EC) and total dissolved solids (TDS).
The concentration of carbonate and bicarbonate species in a particular water source are the primary contributors to its alkalinity. Higher concentrations of these anions increase the inherent buffering capacity of the water, resulting in the water being more resistant to pH correction.
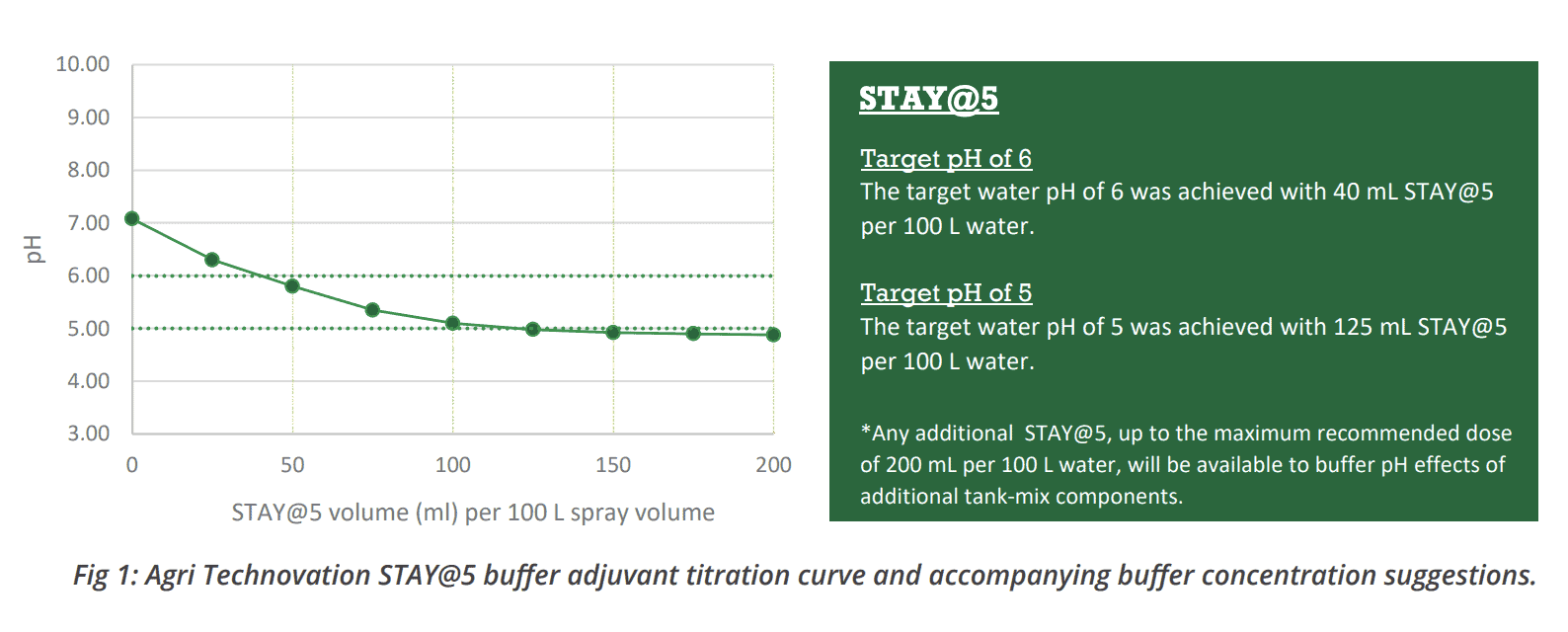
In addition to the pH and alkalinity measurements provided by this analysis, experimentally generated titration curves are displayed alongside suggestions for the required buffer adjuvant concentration to reach specific target pH values (Figure 1).
This is especially important to take into consideration when the application of agrochemicals sensitive to alkaline hydrolysis is concerned, as in these cases it is essential that the water pH be corrected to a value around 5 before their introduction into solution.
The EC and TDS values provided by this analysis are closely tied to the hardness of the water sample and an accurate measure of its mineral and ion content. By virtue of these values, classification of the water source in terms of hardness is inferred along with the extent of salt antagonism expected if susceptible crop protection products are to be applied. Furthermore, calculations are undertaken in order to provide the exact required dosage of AMSure, Agri Technovation’s liquid ammonium sulphate adjuvant, to bind and remove the antagonistic cations in solution.
Conclusion
Spray water analysis has the ability to provide invaluable information to assess the suitability of any water source for tank-mix purposes. Having an understanding of the water quality directly influences the efficacy of the products being applied by ensuring that appropriate measures are taken when the water quality is less than ideal. In addition, suggestions for the most efficient use of adjuvant products save time and money by removing the uncertainty associated with the requirements for water quality correction.





