The crucial role of formulation in the efficacy of nutrient foliar products
By Rochelle Thuynsma, Head of Products: Technical and Dr. Elmi Lötze, Head of ITEST™CARBOHYDRATES and ITEST™LEAF
Introduction
Foliar-applied fertilisers have become an integral and significant part of sustainable and productive crop management worldwide. Successful application of nutrients in permanent crops has been reported for different crops and nutrients i.e., avocado, citrus and apple, with amongst others, copper (Cu), boron (B), calcium (Ca), zinc (Zn) and nitrogen (N).(1-6)
Various techniques are applied to quantify the increase in uptake, penetration depth of the element into the tissue and speed of penetration.(3, 4) However, the efficacy of foliar applied nutrients is governed by various factors such as the physical and chemical properties of the leaf surface, the spray solution, environmental conditions and plant physiology (phenological stage).
These factors all interact to impact the final uptake, translocation and assimilation of nutrients, and must therefore be taken into account when selecting a foliar product.
Product formulation
Commercial foliar products are manufactured differently to address the factors associated with uptake, translocation and assimilation of nutrients. The chemical properties of these products that affect the uptake and or distribution efficiency of nutrients include the molecular size, solubility, electric charge and pH of the applied nutrient and solution.
Molecular structure and chemistry
Larger-sized molecules will be taken up slower due to the small selective permeability of the cuticle, as well as the small size of leaf cracks, pores and ridges.(7) Nonpolar materials pass through plasma membranes more easily than polar materials, allowing a faster rate of diffusion. Polar materials usually require transport against a concentration gradient through ATPdependent carriers(8) – thus requiring additional energy. Similarly, highly charged molecules will more easily bind to leaf surfaces than diffuse into plant cells – reducing uptake. Finally, the pH of the solution affects the efficacy of the products. Ionisable groups vary in their ability to cross cell membranes depending on the pH of the solution, which will affect the predominant charge of molecules.(7)
Leaf assimilation
Nutrient uptake via the cuticle is very limited due to the high hydrophobicity and small molecule size (< 50 nm) required to enter the cuticle directly.
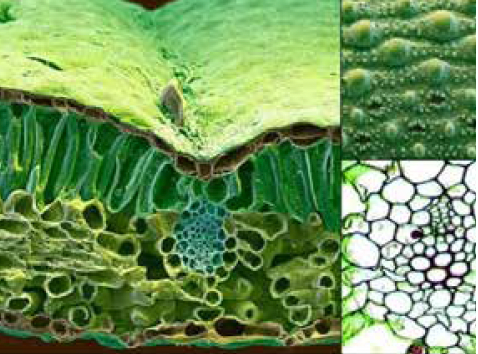
Figure 1: The coupled trans-cellular pathway where the nutrient is deposited in the apoplast and taken up by the adjacent cell via passive (diffusion/facilitated diffusion) or active (ATP required) transport mechanisms.
Therefore, most solutes penetrate through stomata, pores, cracks or ridges (10 to 100 μm), following a diffusion pathway along the opening walls, ultimately moving through the apoplast (intercellular spaces) until reaching the vascular bundle.(7) Another form of uptake involves localised cell uptake and the transfer of nutrients from cell to cell via plasmodesmata – symplastic uptake. The final mechanism for leaf nutrient uptake is the coupled trans-cellular pathway. The nutrient is deposited in the apoplast and taken up by the adjacent cell via passive (diffusion/facilitated diffusion) or active (ATP required) transport mechanisms(8) (Figure 1).
Salts
Straights are based on metal salts, consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. However, salts are water-soluble and dissociate in water into the constituent positive cation and negative anion.
Due to leaf cuticle chemistry (net negative charge), positive cations will interact with the negatively charged carboxyl and hydroxyl groups on the leaf surface, fixing the cation into place on the leaf surface and leaving the nutrient unavailable for plant uptake. Therefore, the concentration of the applied metal salt needs to be high to ensure nutrient availability after leaf fixation. High concentrations of metal salt applications may also lead to phytotoxicity symptoms such as leaf burn.
Chelation
Chelation and complexing reactions are two chemical processes used to produce nutrients linked to organic molecules.(9) Many foliar nutrients like iron (Fe), magnesium (Mg) and Ca are chelated to ensure plant nutrient uptake, reduce fixation/immobilisation and increase final assimilation.
Chelation refers to the molecular binding of a metal/ion to an organic molecule. This is a strong binding with the metal/ion bound within a larger ring structure, for example, EDTA. Complexation refers to the binding of a metal/ ion to an organic molecule via weaker binding forces, to one or several ionic groups on the compound – where the metal/ion can remove itself more easily from the binding (9) to be plant available.
Although chelates increase uptake efficiency, they may reduce assimilation due to the large complex, ringed molecules traditionally used.
These compounds require extensive enzymatic processing and ATP input to access the applied nutrient and to reduce the size of the molecule for phloem loading/unloading.(10)
Complexing
Complexing agents, such as amino acids, organic acids and polysaccharides, increase uptake efficiency and enhance plant assimilation. These compounds are much smaller molecules that easily enter through the cuticle and stomata, have no net charge and therefore optimally diffuse through the leaf structure.
The organic nature of these compounds also ensures easy phloem loading and unloading, reducing ATP cost for nutrient assimilation and increasing nutrient use efficiency.(11) The resulting molecules, after nutrient cleavage, can feed directly into plant metabolism, helping to replenish various intermediates in plant metabolism and also serve as a source of carbon (C) and N for plant growth.
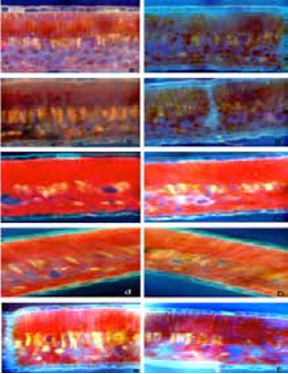
Figure 2: Photographic sequence of cross-sections in avocado leaves. Cuts observed under fluorescence microscopy (40X) illustrate the behaviour of the solution applied. Cuts 15 min (a), 30 min (b), 120 min (c), 240 min (d) and 480 min (e) after the foliar application of distilled water. Cuts 15 min (A), 30 min (B), 120 min (C), 240 min (D) and 480 min (E) after foliar application of calcofluor-boron solution.
They furthermore stimulate plant growth, may help to alleviate the effects of stress on the plant and have natural wetting/ spreading properties. (12) In addition to the spray solution properties and nutrient source, the following plant and environmental factors also influence foliar efficacy as a nutrient source.
Leaf surfaces and chemical properties
The cuticle has a net negative charge to the leaf surface (12) and, when observed under the microscope, shows ridges, cracks, pores, stomatal openings and (in certain plant species) trichomes(8)(Figure 1).
These cuticular structures provide entry points for foliar-applied nutrients to reach the cellular leaf structures below the surface (Figure 2).
The leaf development stage impacts the uptake and distribution of nutrients. Immature leaves function as a sink and are incapable of exporting nutrients until they have matured.(13) It is therefore critical to apply foliar applications at the correct phenological stages with adequate leaf area, to ensure effective uptake and distribution of foliar-applied nutrients within the plant.
Phloem loading and unloading (leaf assimilation) transport and distribute nutrients throughout the plant, through the symplast or apoplast.(13)
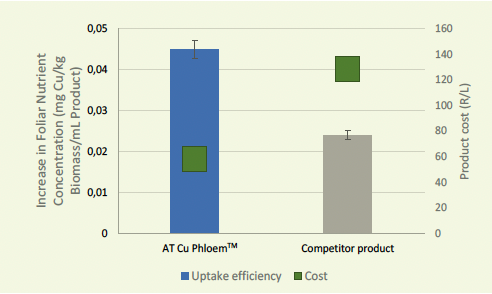
Figure 3: Cu Phloem™ vs. competitor product water-soluble fraction of Cu in leaves applied on lettuce.
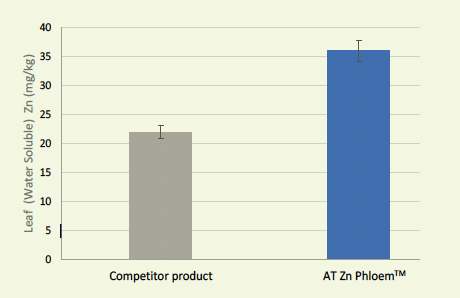
Figure 4: Zn PhloemTM vs Competitor Product, water soluble leaf fraction of macadamia, 10 days after application.
Nutrients need to be complexed with organic molecules to facilitate phloem loading and unloading.(13) Passive phloem loading involves solutes diffusing freely with the pressure gradient through the symplast. Active phloem loading involves an ATP-driven process, usually coupled with apoplastic transport. When nutrients reach the sink organ they are unloaded from the sieve-tube elements to the receiving cells.
Environmental factors
Environmental factors influence foliar uptake efficacy directly by affecting the efficacy of the spray solution(12), or indirectly by altering photosynthesis, stomatal opening, respiration, leaf expansion and source/sink activity.(8)
Light signals play a crucial role in regulating N uptake, translocation and assimilation into organic compounds. (14) Higher light intensity can increase nutrient uptake by enhancing plant photosynthesis and sugar accumulation.
Temperature can affect the chemistry of the spray solution, the leaf surface(12), the movement of molecules within the spray solution, as well as the rate of nutrient uptake. High temperatures also increase the drying time of foliar-applied nutrients, thereby reducing uptake(10) and as a response to high temperatures, plants will close their stomata to prevent water loss, further decreasing foliar uptake. Low temperatures can reduce nutrient uptake by decreasing the plant growth rate, resulting in reduced nutrient demand and root respiration. (12)
High relative humidity favours nutrient uptake through leaves by decreasing the drying rate and causing the cuticle to swell as it absorbs water from the atmosphere.(12) This results in increased surface pore creation, acting as additional entry points for foliar-applied nutrients.
Plants will also preferentially open their stomata under high humidity conditions, further aiding nutrient absorption.(11)
Conclusion
The complexed PHLOEM™ Agri Technovation product range addresses all the concerns around foliar nutrient uptake, movement and assimilation. The PHLOEM™ range is organically complexed with amino acids, organic acids and carbohydrates to ensure effective leaf uptake and nutrient use (Figures 3 and 4).
These complexing agents not only facilitate uptake and transport, but also help to alleviate the effects of environmental and physiological stress factors. Amino and organic acids are well-known alleviators of stress, allowing the opening of stomata to ensure efficient nutrient uptake, while supporting phloem loading and unloading, and ultimately feeding into various plant metabolic processes to support growth.
Carbohydrates contribute to plant energy metabolism and can be used directly or fed into multiple metabolic processes to regenerate co-factors and carbon skeletons.
The PHLOEM™ range is thus expertly designed to ensure efficient nutrient delivery and assimilation and overcome the limitations usually associated with foliar products.
ZINC PHLOEM™ Reg. no: B5734.
References:
- Izadyar, A.B., Malakouti, M.J., Talaie, A.R. and Fallahi, E. 1998. Biennial bearing and protein content of apples as influenced by high concentrations of foliar nitrogen and sulfur. Journal of Plant Nutrition. 21:649–653.
- López-Luna, J., Nopal-Hormiga, Y., López-Sánchez, L., Mtz-Enriquez, A.I. and Pariona, N. 2023. Effect of methods application of copper nanoparticles in the growth of avocado plants. Science of The Total Environment Vol. 880, 163341.
- Lötze, E., Wilsdorf, R.E., Turketti, S.S., Przybylowics, W.J., Mesjasz-Przybylowicz, J., 2015. Revisiting Ca concentration and distribution in apple fruit (Malus domestica): Comparing transverse results from PIXE, SEM and destructive mineral analyses. Journal of Plant Nutrition 38: 1469-1477.
- Lötze, E., Turketti, S., 2015. Efficacy of foliar application of calcium products on tomatoes as defined by penetration depth of and concentration within fruit tissues. Journal of Plant Nutrition 38: 1-14.
- Lovatt, C. 2013. Properly Timing Foliar-applied Fertilizers Increases Efficacy: A Review.
- Update on Timing Foliar Nutrient Applications to Citrus and Avocado. Horttechnology 23(5).
- Torres, M.D., Farré, J.M. and Hermoso, J.M. 2002. Foliar B, Cu and Zn Applications to Hass Avocado Trees. Penetration, Translocation and Effects on Tree Growth and Cropping. Acta Hort. 594.
- Riederer, M., Friedmann, A. 2006. Transport of lipophilic non- electrolytes across the cuticle, in Annual Plant Reviews, Biology of the Plant Cuticle, Eds M. Riederer and C. Müller (Oxford: Blackwell) Vol.23: 250–279.
- Fernández, V., Eichert, T. 2009. Uptake of hydrophilic solutes through plant leaves: current state of knowledge and perspectives of foliar fertilization. Critical Reviews in Plant Science Vol 28: 36.
- Rodríguez-Lucena, P., Hernández-Apaolaza, L. and Lucena, J.J. 2010. Comparison of iron chelates, and complexes supplied as foliar sprays and in nutrient solution to correct iron chlorosis of soybean. Z. Pflanzenernähr. Bodenk Vol. 173: 120.
- Fageria, N.K., M., Barbosa Filho, M.P., Moreira, A., Guimarães, C.M. 2009. Foliar Fertilization of Crop Plants. Journal of Plant Nutrition Vol. 32: 1044.
- Alshaal, T., El-Ramaday, H. 2017. Foliar Application: from Plant Nutrition to Biofortification. Journal of Environmental Biodiversity and Soil Security Vol.1: 1.
- Fernández, V., Brown, P. H. 2013. From plant surface to plant metabolism: the uncertain fate of foliar-applied nutrients. Frontiers in Plant Science Vol 4: 289.
- Turgeon, R. 2006. Phloem loading: How leaves gain their independence. Bioscience. Vol 56: 15.
- Xu, J., Guo, Z., Jiang, X., Ahammed, G.J., Zhou, Y. 2021. Light regulation of horticultural crop nutrient uptake and utilization. Horticultural Plant Journal Vol. 7: 367.





