Dr. Jakkie Stander, Commercial Head of Products
Rochelle Thuynsma, Head of Products: Technical
Calcium is vital for maintaining cell-wall structure, where it cross-links pectins in the middle lamella, as well as for membrane integrity and signalling processes. However, when applied foliarly, Ca faces several physiological and physical bottlenecks.
Because Ca is transported predominantly via the xylem and has very limited phloem mobility (White, 2001), developing fruit – which often have weak transpiration streams – are poorly supplied. After entry, Ca²⁺ strongly binds within the apoplast, limiting redistribution and movement into deeper tissues (Hocking et al., 2016).
Furthermore, the cuticle and epidermis present substantial barriers to penetration, especially given that ionic solutes like Ca²⁺ require extended surface hydration and specific polar pathways to diffuse effectively (Fernández & Brown, 2013). These limitations explain why the efficacy of foliar Ca sprays often depends on repeated applications, direct fruit coverage and favourable environmental conditions that prolong droplet wetness.
Pathways for foliar calcium uptake
The entry of Ca into leaves and fruit is governed by droplet retention, hydration time and cuticular microstructure. Prolonged droplet wetness promotes diffusion of ions through hydrophilic domains of the cuticle and epidermal cell walls. Stomatal uptake may contribute in leaves but is of limited relevance in fruit skins, where stomata become non-functional as development progresses (Eichert & Goldbach, 2008).
In fruit, uptake occurs primarily through polar micro-domains, surface microcracks and the pedicel-fruit junction, all of which have been identified as Ca absorption hotspots (Winkler et al., 2021; Hurtado et al., 2025).
These findings emphasise that direct sprays onto fruit surfaces, particularly at sensitive entry points, are critical for effective Ca delivery.
Mechanistic rationale for a calcium-pectate formulation
A calcium-carbohydrate complexed formulation such as CALCINATOR™ is designed to overcome many of the shortcomings of conventional calcium sprays (Fig. 1). In this system, Ca²⁺ ions are complexed with low-molecular-weight carboxylates, producing a buffered and hydrophilic complex with distinctive physico-chemical properties. The gel-forming nature of pectic ligands increases droplet viscosity and humectancy, which enhances adhesion to the fruit skin, reduces droplet rebound and prolongs hydration time (Blanco et al., 2010).
This extended wetness improves the window for cuticular penetration. The ligand also buffers free Ca²⁺ activity, reducing the tendency of calcium to bind immediately and irreversibly to negative charges on the fruit surface (Fernández & Brown, 2013).
By moderating ionic activity at the interface, more Ca can diffuse into the apoplast before becoming immobilised. Once inside, Ca²⁺ is gradually released from the complex and incorporated into endogenous homogalacturonan chains within the middle lamella, forming calcium-pectate cross-links known as the “egg box” structure. These cross-links strengthen the cell wall, reduce membrane leakage and slow down pectin solubilisation, thereby improving fruit firmness and reducing physiological disorders (Huang et al., 2023).
Compared with highly soluble salts like CaCl₂, which deliver high osmotic loads, the CALCINATOR™ formulation delivers Ca more gently, reduces salt burn risk and offers broader compatibility in tank mixes (Wójcik et al., 2014).
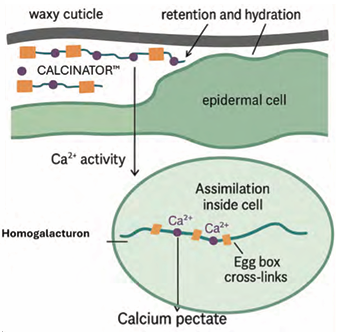
Figure 1. Uptake and incorporation of CALCINATOR™ into fruit cell wall.
Comparison with other calcium sources
Non-complexed calcium salts such as CaCl₂ and Ca(NO₃)₂ are effective at increasing fruit Ca content when applied under favourable conditions. However, they are associated with significant limitations, including high phyto-toxicity risk, particularly under warm, slow-drying conditions, and frequent incompatibility in tank mixes due to precipitation with phosphates and sulphates (Autio & Schupp, 2001; Wójcik et al., 2014).
While they may deliver high amounts of Ca quickly, their performance is inconsistent and highly dependent on environmental conditions. Suspension concentrates (SCs) of sparingly soluble Ca sources such as CaCO₃ act differently. These formulations deposit particles on the leaf or fruit surface, where gradual dissolution releases Ca over time.
While SCs can provide reflective and protective benefits that indirectly support fruit physiology, their nutritional contribution is limited by the slow dissolution rates of these compounds under field conditions (Pimentel et al., 2023). In contrast, CALCINATOR™ provides both rapid and biochemically relevant assimilation of Ca into fruit tissues, prioritising direct nutritional effects over surface conditioning.
Uptake, translocation and assimilation of calcium-pectate
The uptake process of CALCINATOR™ begins with the deposition of droplets that adhere strongly to the fruit surface and remain hydrated for extended periods. Within these droplets, buffered Ca²⁺ complexes diffuse through polar cuticular pathways and microcracks. Once inside the epidermis and apoplast, translocation occurs primarily over short distances, as Ca remains largely immobile in the symplast and phloem (White, 2001).
This local distribution underscores the importance of repeated direct fruit sprays. The assimilation step is critical: As the Ca²⁺ ions dissociate from the ligand, they bind to homogalacturonan blocks in the cell wall, forming calcium-pectate cross-links that increase firmness and resistance to breakdown (Huang et al., 2023).
Conclusion
Calcium-pectate foliar formulations such as CALCINATOR™ represent a scientifically rational approach to addressing the persistent challenges of foliar calcium nutrition in fruit crops. By coupling prolonged droplet retention and buffered ionic activity with targeted assimilation into cell-wall pectins, these formulations improve efficacy while reducing salt burn risk and broadening compatibility.
Compared with non-complexed salts, they deliver Ca more gently and reliably, while offering stronger nutritional benefits than suspension concentrates. Ultimately, success still depends on repeated direct fruit coverage and favourable environmental timing, but calcium-pectate formulations represent a meaningful advancement in optimising fruit Ca status and reducing calcium-related disorders.
Calcinator™ Fertilizer group 2 | Reg. number b5022 | Act 36 of 1947
References:
1. Autio W, Schupp J (2001/2012). Foliar calcium sprays for apples (F-119R). Univ. of Massachusetts / Univ. of Vermont.
2. Blanco A, et al. (2010). Improving the performance of calcium-containing spray formulations. Scientia Horticulturae 125.
3. Eichert T, Goldbach HE (2008). Equivalent pore radii of hydrophilic foliar uptake routes. Plant, Cell & Environment 31:1299–1309.
4. Fernández V, Brown PH (2013). From plant surface to plant metabolism. Frontiers in Plant Science 4:289.
5. Hocking B, Tyerman SD, Burton RA, Gilliham M (2016). Fruit calcium: transport and physiology. Frontiers in Plant Science 7:569.
6. Huang W, et al. (2023). Calcium-mediated homogalacturonan complexation contributes to firmness in loquat. Journal of Advanced Research 49:47–62.
7. Hurtado G, et al. (2025). Pathways and factors in calcium uptake through strawberry skins. Scientific Reports 15.
8. Hu Y, et al. (2023). Editorial: Factors affecting the efficacy of foliar fertilizers. Frontiers in Plant Science 14:1121806.
9. Pimentel C, et al. (2023). Mineral particles in foliar fertilizer formulations. Plants 12.
10. White PJ (2001). The pathways of calcium movement to the xylem. Journal of Experimental Botany 52:891–899.
11. Winkler A, et al. (2021). Calcium uptake through skins of sweet cherry fruit. Scientia Horticulturae 283:110059.
12. Wójcik P, et al. (2014). Preharvest CaCl2 sprays on apple quality and leaf injury. Scientia Horticulturae 171:46–51.
13. Yamane T (2014). Foliar calcium applications in deciduous fruit trees: an overview. JARQ 48(1):25–31.





